Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Research Article(ISSN: 2638-5910) 
Plukenetia conophora L (African Black Walnut) Consumption Improves Diabetes Control in Experimental Diabetic Rats without Influence on Body Weight Change Volume 4 - Issue 3
Magnus Michael Chukwudike Anyakudo* and Moyinoluwa Bolanle Oyeniyi
- Endocrinology, Metabolism and Clinical Nutrition and Applied Research Unit of Department of Physiology, Faculty of Basic Medical Sciences, University of Medical Sciences, Nigeria
Received:March 15, 2023; Published:March 31, 2023
Corresponding author:Magnus Michael Chukwudike Anyakudo, Metabolism and Clinical Nutrition and Applied Research Unit of Department of Physiology, University of Medical Sciences, Ondo State, Nigeria
DOI: 10.32474/ADO.2023.04.000190
Abstract
Background and Objectives:Conflicting data and reports exist on walnut effects on weight management and diabetes control. This experimentally-controlled nutritional study examined the effects of African black walnut (Plukenetia conophora) on weight and diabetes control in experimental diabetic male Wistar rats. The impacts of African black walnut on body weight gain, glycemic tolerance and lipoglycemic profile were evaluated in this study.
Materials and Methods:Three experimental groups (n = 10, each) of adult male Wistar rats weighing 170-220g were randomly categorized after induction of diabetes with alloxan (150mg/kg b.w; i.p): Control (group DCR); Extract-treated (group AWE) and, Diet-supplemented (group BSW). Animals were fed according to the experimental design with water ad libitum for six weeks. Body weight, total food intake (TFI) per group, food conversion ratio (FCR), fasting blood glucose (FBG) concentrations, lipid profile (LP) and, oral glucose tolerance test (OGTT) were determined and conducted. Data were analyzed using Microsoft Excel and Statistical Program SPSS version 22. Results are expressed as mean ± SEM with a P value set at 0.05.
Results:Black walnut consumption caused a significant (P < 0.05) reduction in mean FBG concentrations (AWE: 25.95%; BSW: 21.07%) compared with the control group. The glycemic tolerance and lipid profile were improved significantly by black walnut in AWE and BSW rats compared with the control rats without corresponding change in body weight, TFI and FCR. The aqueous extract impacted more lipoglycemic effect than the diet-supplemented.
Conclusion:Plukenetia conophora improves diabetes control in diabetic rats via its beneficial lipoglycemic impact without a corresponding influence on body weight change.
Introduction
Walnuts are among the most widely consumed commercially grown tree nuts in the world [1]. However, the relative scarcity of wide-scale molecular phylogeographic studies of walnut makes it difficult to accurately determine the native geographic range. Plukenetia conophora, also called African walnut, and conophore, is a climbing shrub with highly versatile nut native to tropical western and central Africa and abundant in Nigeria, Cameroon and Congo [2]. In Nigeria, it is locally called Asala or Awusa in Yoruba; Ukpa in Igbo; Gyada in Hausa and Okhue or Okwe in Edo. It belongs to the botanical family Euphorbiaceae [3]. Many claims of health-promoting benefits have been attributed to walnut consumption which include preventing, treating and alleviating risk of cardiovascular disease [4-6], type II diabetes [7,8], obesity [9-13], certain cancers [14], and the alleviating of symptoms attributed to age-related and other neurological disorders [15]. These health benefits are ascribed to its rich antioxidants and fatty acid profile, which is rich in polyunsaturated fatty acids with a particularly high ω3:ω6 ratio - the highest among all the tree nuts [16]. Most experts agree that consuming nuts, including black walnuts, has well-established links to improving cardiometabolic profile and overall health.
A large cohort study by Jiang et al. [7] and Pan et al. [8] showed that dietary supplementation of walnuts significantly decreased the risk of developing type 2 diabetes while a similar observation was also reported by Ros [4] in a study of long-term intervention with a Mediterranean diet enriched with nuts. Considering the large number of people with diabetes and the growing projected numbers, there is an urgent need to develop and implement coordinated and multi-sectoral strategies to tackle diabetes mellitus. The content of polyphenols and other phytochemicals including phospholipids, vitamins, minerals, essential fatty acids, and other nutrients in walnuts, with their claimed cytotoxic properties, also make them a focus of attraction for research for the prevention of free radical-induced nucleic acid damage. Research of walnut consumption in humans and animals employing a range of data sets and statistical methods suggest that walnuts may be considered a safe potential nutraceutical or possibly pharmaceutical substance [17].
A study [18] claimed that walnut is helpful in weight management and declared it as a nut of choice for obese and diabetic individuals. Contrary to expectations, clinical trial, epidemiological studies and, systematic reviews of different studies have shown that walnut consumption in the diet does not contribute to weight gain or hinder weight loss goals as compared to a control diet [6,11,18,19]. To help resolve this controversy polarizing the opinions of leading experts due to conflicting data and reports existing on walnut health benefits on weight management and diabetes control, this study was conducted to investigate the effects of aqueous extract and diet-supplementation of African black walnut (Plukenetia conophora) on body weight gain, total food intake, food conversion ratio, glycemic tolerance/control and, lipoglycemic profile in diabetic male Wistar rats with the rationale to provide a more objective basis to guide dietary recommendations of walnut in diabetes control (Figures 1-3).
Materials and Methods
Preparation of Plukenetia conophora aqueous extract and diet-supplemented
The fresh black walnut (Figure 1) was purchased from a local popular market in Ondo city of Southwest of Nigeria with the help of an agriculturist who identified the species. Extract preparation was made by neatly cracking and exposing the seed to remove the pellicle and then pounded finely with mortar and pestle. The pounded walnut was sun dried and then pounded to a homogenous powdery form. 4g of powdery walnut was mixed with 20mls of tepid distilled water to obtain the aqueous extract fraction (via filtration method) which was kept at 4°C in the refrigerator until required. Fresh aqueous walnut extract was prepared each week of the study period. The supplemented diet was composed and prepared as reported under the heading ‘Composition of control and test diets’.
Toxicity Test
Acute toxicity test of the extract was conducted using modified stair-case approach of Chinedu et al. [20]. The rats were given escalating dosages (1, 2, 3, and 4g per kg body weight) of extract of fresh walnut orally using orogastric canula while the toxicity was determined by mortality and behavioral abnormalities. The peak tolerance level was at 2g/kg body weight.
Composition of Test and Control Diets
Using the standard diet formula used to assess weight gain in rodents during commercial feeding studies, the composition of the supplemented and control diets was constituted (Table 1). The control (normal ration) and the supplemented diets were prepared from ingredients purchased from a popular market in Ondo Metropolis, Ondo State, Nigeria and are expressed in percentage per 100 g feed. The walnuts were boiled and mashed with the ingredients to constitute the diet-supplemented.
Experimental Animals and Feeding
The animals were purchased from disease-free stock of Ade farm, Ogbomoso, Oyo State, Nigeria. Twenty-one male albino Wistar rats (Rattus norvegicus) weighing between 170g and 220g were used for this investigation. The animals were kept in polypropylene cages with stainless wire mesh tops in a well ventilated animal house for two weeks acclimatization at normal and standard laboratory temperatures and relative humidity. They were fed initially with commercially available standard rat feed and water ad libitum during acclimatization and thereafter, fed according to the experimental design for 6 weeks. Group AWE in addition to the standard feed was treated with aqueous extract at daily tolerable dose of 2g/ kg body weight determined by acute toxicity test conducted while group BSW was fed with walnut-supplemented diet only with daily serving size of 2g/kg body weight (equivalent to 200g of walnut per kg of feed) based on the total weight of the number of rats per group to ensure no differential calories treatment in both control and test diets. Body weight and total food intake of each group of rats were measured and recorded weekly while the food conversion ratio (food intake/weight gain) was calculated. Under each cage, replaceable numbered blotter papers were placed to catch the spilled diet which was measured to make up for the daily serving ration.
Ethical Approval
This study using experimental animals was conducted in strict accordance with the internationally accepted principles and recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health [21]. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Medical Sciences Ondo (Protocol Number REC/27042021).
Induction Of Diabetes
After acclimatization, all rats were inducted with diabetes after overnight fast using fresh solution of alloxan monohydrate (Kermel chemicals, CHINA) made by dissolving it in sterile normal saline at a dose of 150 mg/kg body weight. A glucometer (Fine test Auto-coding Premium Blood glucose Monitoring System, Osang Healthcare Co., Ltd. Korea) was used to determine the FBG level and confirm diabetes four days later. Rats with FBG ≥150 mg/dL were considered diabetic and used for the study.
Experimental Design
The animals were randomly categorized into three groups (n = 7/group):
a) Group AWE: Diabetic rats treated with walnut aqueous extract b) Group BSW: Diabetic rats treated with boiled walnut-supplemented diet c) Group DCR: Diabetic control Rats in group DCR were fed with standard rat feed (SRF) only throughout the period of the study. Group AWE rats were treated with aqueous extract of walnut (2g/kg body weight/day, orally) plus SRF while group BSW rats were fed walnut-supplemented diet only. The rats were monitored twice daily for food and water intake while body weight and FBG were assessed bi-weekly and recorded.
Blood Collection and Biochemical Assay
The blood samples were obtained from the tail veins (via Snipping) and the heart (through cardiac puncture technique) using fine hypodermic needle (size 23G) and were used to assay the glycemic status/tolerance and lipid profile respectively. All efforts were made to minimize pain and suffering.
Glycemic Tolerance Test
Animals in all groups were fasted overnight (12 hours) with free access to water and their FBG determined in the morning of the day for OGTT. Thereafter, they were treated with an oral D glucose load of 2gm kg-ˡ (dissolved in distilled water) administered by means of flexible orogastric canula. Blood samples were then withdrawn from the tail vein of each animal to determine the FBG concentration at intervals of 30 minutes for 2-hour duration.
Lipid Profile Test
The lipid profile was conducted at the commencement and the
6th week of the study (to enable comparison of values) using blood
samples obtained from the heart stored in k3 EDTA (Ethylene Diamine
Tetraacetic Acid) sample bottles. Samples were centrifuged
at 3000 revolutions to obtain the plasma fractions which was kept
in a refrigerator (at -70ºC) until used and the sera obtained were
used for the biochemical assay of the lipid profile. Plasma concentration
of total cholesterol (TC), high density lipoprotein (HDL)
and Triacylglycerol (TAG) were measured by the enzymatic colorimetric
method after centrifugation using a dry-chemical automatic
analyzer AU-5200 OLYMPUS (Randox Laboratories, San Francisco,
USA). LDL level was determined by the Friedewald formula [22] as
follows:
VLDL (mg/dL) = TAG/5 (1)
LDL (mg/dL) = TC - VLDL – HDL (2)
Statistical Analysis
Data was analyzed using Microsoft Excel and Statistical Pro gram SPSS version 22. Graph pad prism while comparison between groups was made with one way analysis of variance (ANOVA) followed by Duncan’s multiple range tests. Results are expressed as mean ± SEM with P value set at 0.05.
Results
Effects of black walnut consumption on total food intake and body weight
Values are expressed in mean ± SEM.
Values are expressed in mean ± SEM,
**Significant when compared with DCR but not significant when compared with BSW.
*Significant when compared with DCR.
The effect of black walnut on overall body weight and food intake is expressed in Table 2. Consumption of black walnut as supplemented- diet or as raw nut has no effect on overall mean body weight gain and food intake. There was no interaction of diet and time over the 6-week period as revealed by the repeated measures ANCOVA using the total food intake for each animal as a co-variable. No significant difference observed in the food conversion ratio (food intake/weight gain) between groups.
Effects of black walnut consumption on glycemic status
Both walnut-supplemented diet and walnut extract exhibited significant (P < 0.05) hypoglycaemic potential. Difference in their hypoglycaemic potentials is not significant (P > 0.05). However, the extract caused more hypoglycemic effect than the supplemented (AWE - 25.95%; BSW - 21.07%) as shown in Table 3.
Effects of black walnut consumption on glycemic tolerance and profile
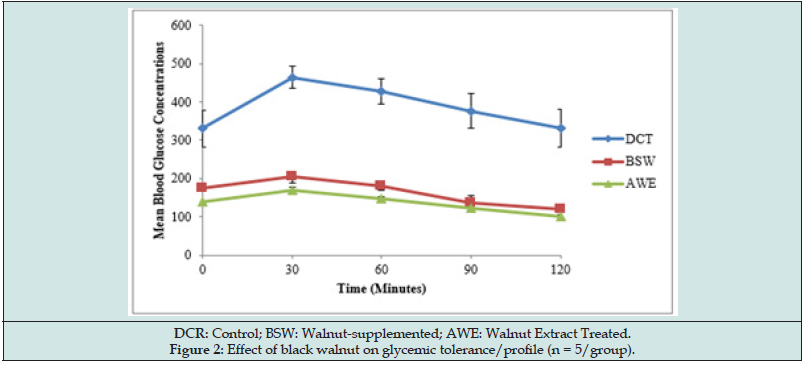
The glycemic tolerance was assessed by the incremental areas under the glycemic response curves as depicted in Figure 2. Both walnut-supplemented and walnut extract improved glycemic tolerance and profile with the extract posing better tolerance and profile. The glycemic response peaked at 30 minutes of the glucose challenge in all experimental groups.
Effects of black walnut consumption on lipid profile
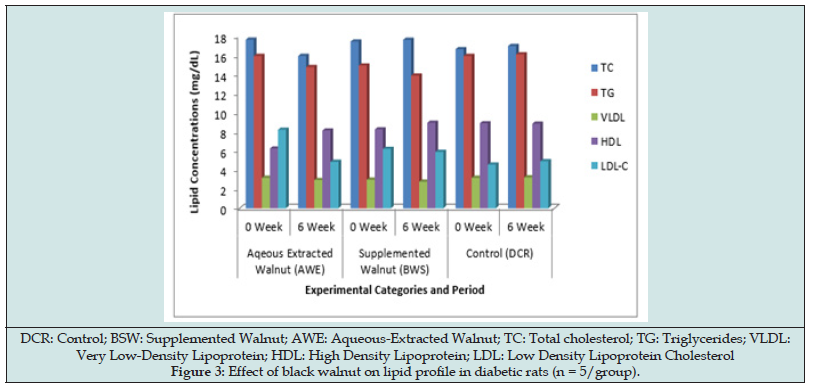
Figure 3 displayed the effect of black walnut on the lipid profile parameters. Both the walnuts supplemented and extract possessed antilipidemic property expressed by their impact on the lipid parameters. However, the walnut extract exhibited more antilipidemic potential (P > 0.05). At the end of the study, the TC, TG and LDL concentrations decreased significantly (P < 0.05) with a corresponding increase (P < 0.05) in HDL concentration in AWE and BSW groups compared with the control.
Discussion
This nutritional study examined the effects of aqueous extract and diet-supplementation of African black walnut (Plukenetia conophora L.) in diabetes control by investigating its impacts on body weight, glycemic tolerance and lipoglycemic profile in diabetic male Wistar rats. Mode of consumption of the black walnut for optimal benefits in diabetes control was also investigated. Main findings revealed that walnut has significant hypoglycemic and antilipidemic effects helpful in diabetes control without corresponding influence on weight control. These effects were impacted more by the extract than the diet-supplemented.
Walnut was processed and administered in two forms (aqueous extract and diet-supplemented) in this study. Both forms have no influence on mean body weight gain, total food intake and food conversion ratio in the test groups compared with the control (Table 2). This observation suggests that walnut has no effect on the appetite which may warrant increased food intake that may influence weight change. A study [18] claimed that walnut-enriched low-energy diet is helpful in weight management and so declared walnut as a nut of choice for obese and diabetic. However, contrary to expectations, some clinical trials, epidemiological studies, and systematic reviews of other studies [6,11,19] showed that walnut consumption in the diet does not contribute to weight gain or hinder weight loss goals.
Table 3 expressed the hypoglycemic impact of black walnut in experimental diabetic rats. Both forms of walnut exhibited significant (P < 0.05) hypoglycaemic potentials. Walnut extract caused 25.95% decrease in mean FBG concentration while the diet-supplemented decreased mean FBG value by 21.07%. This antidiabetic benefit of walnut in diabetic rats suggests that eating walnuts may help control blood sugar by mechanisms beyond their influence on weight control. Human and animal studies have shown that eating walnut may help control blood sugar in diabetic or pre-diabetic individuals: A human study [23] revealed that consumption of walnut oil (15 g/day for three months) improved blood glucose level without changes noted for bodyweight and blood pressure in type two diabetic patients. Dietary supplementation of 1 oz of nuts, such as walnuts, five times or more per week in another human studies [7,8] has been revealed to decrease risk of developing type 2 diabetes. A long-term intervention study with Mediterranean diet enriched with nuts has also reported an association of nuts with a 50% reduction in diabetes [4].The antidiabetic property of walnut was further heightened by its improved glycemic tolerance and profile in the diabetic rats as depicted by the decrease in the incremental areas under the glycemic response curves for BSW and AWE rats compared with the control (Figure 2). This glycemic tolerance effect in AWE rats is comparably better than those in BSW rats. Glycemic response to glucose load in all the experimental groups peaked at 30 minutes of the glucose challenge suggesting that the blood sugar lowering effect of walnut can become manifest 30 minutes after meal.
On lipid profile, black walnut caused significant decrease in TC, TG and LDL-C concentrations with corresponding significant increase in HDL concentration in both AWE and BSW rats (Figure 3). However, the aqueous extract impacted more beneficial antilipidemic effect than the walnut-supplemented diet thus suggesting that the optimal effect on lipid profile can be derived when walnut is consumed in fresh or raw form than in mixed meal. Walnuts have been reported to have higher antioxidant activity than any other common nut. The papery skin of walnuts has been demonstrated to contain vitamin E, melatonin and polyphenols [24,25]. A preliminary small study in healthy adults showed that eating a walnut-rich meal prevented postprandial oxidative-damage-causing atherosclerosis due to LDL cholesterol [26]. Ellagitannins, a potent anti- inflammatory polyphenol has been reported to prevent oxidative stress and inflammation. Beneficial bacteria in the gut convert ellagitannins to compounds called urolithins which have been found to protect against inflammation. ALA omega-3 fat, magnesium and the amino acid arginine in walnuts may also decrease inflammation [27]. Few studies [4,5,6] have suggested that walnuts in the diet can reduce the risk of heart disease by improving various cardiometabolic risk factors while several studies [9-13] on walnut diets have reported a decrease in total and low-density lipoprotein (LDL) cholesterol, increase high-density lipoprotein (HDL) cholesterol, and reduce blood pressure, inflammation, and plaque formation.
Due to evidence in support of the benefits of walnuts related to cardiovascular health, most experts agree that consuming nuts, including black walnuts, has well-established links to improving heart health. Nuts, such as black walnuts, are high in fatty acids and antioxidants that can help to improve a person’s overall heart health [28]. A variety of plant-derived and naturally-derived compounds are currently being employed to assist diabetic patients in their therapy. As a result, screening medicinal plants for therapeutic purposes is critical in drug development because they may have hypoglycemic, hypolipidemic, and antioxidant properties that are useful in the control of diabetes.
Conclusion
In conclusion, walnut improved glycemic tolerance and lipoglycemic profile in experimental diabetic male rats without corresponding effect on body weight change. While the optimal effect of walnut in diabetes control is more derived with the extract as shown in this study, however, caution must be applied when consuming raw walnut to avoid unexpected toxicity. Further investigations of walnut in experimental diabetic rats will be developed in our subsequent study in order to evaluate the antioxidant capacity of black walnut and its effects on Insulin level and HOMA-IR index to gain more insight into the mechanism of action of walnut in diabetes control. Pancreas histomorphometry will also be carried out to elucidate the effects of walnut on Islets cells.
Authors Contributions
This work was carried out in collaboration between the authors. Author MMCA designed, supervised, performed the analysis and interpretation of data and wrote the manuscript while author MBO assisted in the provision of essential materials and acquisition of data. Both authors read and approved the final manuscript for submission.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Acknowledgements
Declared none.
References
- Pollegioni P, Woeste K, Chioccine F (2017) Rethinking the History of Common Walnut (Juglans regia L.) in Europe: Its Origins and Human Interactions. PLoS ONE 12(3): 1-24.
- Amusa TO, Jimoh SO, Azeez IO, Awodoin RO, Kareem I (2014) Stock density and fruit yield of African walnut, Plukenetia conophora Mull-Arg (Syn. Tetracarpidium conophorum) in tropical lowland rainforests of southwest Nigeria. Journal of Tropical Forestry and Environment 4 (2): 73-81.
- Croizat L (1945) The tribe Plukenetiinae of the Euphorbiaceae in Eastern tropical Asia. Journal of the Arnold Arboretum 22 (3): 417-431.
- Ros E (2015) Nuts and CVD. Br J Nutr 113 (Suppl. 2): 111-120.
- Kris-Etherton PM (2014) Walnuts decrease risk of cardiovascular disease: A Summary of Efficacy and Biologic Mechanisms. J Nutr 144: 547-554.
- Banel DK, Hu FB (2009) Effects of Walnut Consumption on Blood Lipids and other Cardiovascular Risk Factors: A Meta-analysis and Systematic Review. Am J Clin Nutr 90: 56-63.
- Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC et al. (2002) Nut and Peanut Butter Consumption and Risk of Type 2 Diabetes in Women. JAMA 288: 2554-2560.
- Pan A, Sun Q, Manson JE, Willett WC, Hu FB (2013) Walnut Consumption is Associated with Lower Risk of Type 2 Diabetes in Women. J Nutr 143: 512-518.
- Sabaté J, Cordero-Macintyre Z, Siapco G, Torabian S, Haddad E (2005) Does Regular Walnut Consumption Lead to Weight Gain?. Br J Nutr 94(5): 859-64.
- Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ (2017) Associations between Dietary Patterns and Blood Pressure in a Clinical Sample of Overweight Adults. J Acad Nutr Diet 117(2): 228-239.
- Guasch Ferré M, Li J. Hu FB, Sala Salvadó, Tobias DK (2018) Effects of Walnut Consumption on Blood Lipids and other Cardiovascular Risk Factors: An Updated Meta-analysis and Systematic Review of Controlled Trials. Am J Clin Nutr 108: 174-187.
- Estruch R, Ros E, Salas Salvadó J, Covas MI, Corella D (2018) Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med 378: e34.
- Tindall AM, Petersen Skulas-Ray KS, Richter AC, Proctor CK, Kris-Etherton DN (2019) Replacing Saturated Fat with Walnuts or Vegetable Oils Improves Central Blood Pressure and Serum Lipids in Adults at Risk for Cardiovascular Disease: A Randomized Controlled-Feeding Trial. J Am Heart Assoc 8: e011512.
- Lin CH, Ho KV, Roy A, Foote S, Vo PH, et al. (2020) Profiling Anticancer and Antioxidant Activities of Phenolic Compounds Present in Black Walnuts (Juglans nigra) Using a High-Throughput Screening Approach. Molecules 25(19): 4516.
- Hosseini Adarmanabadi SMH, Karami Gilavand H, Taherkhan, A, Sadat Rafiei SK, Shahrokhi M, et al. (2022) Pharmacotherapeutic potential of walnut (Juglans spp.) in age-related neurological disorders. IBRO Neuroscience Reports 14: 1-20.
- Schlegel V, Câmara S, Rodrigues C (2016) A Review on the Potential Human Health Benefits of the Black Walnut: A Comparison with the English Walnuts and Other Tree Nuts. International Journal of Food Properties 19(10): 2175-2189.
- Chauhan A, Chauhan V (2020) Walnut consumption in a weight reduction intervention: effects on body weight, biological measures, blood pressure and satiety. Nutrition Journal 12: 550.
- Rock CL, Flatt SW, Barkai HS, Pakiz B, Heath DD (2017) Walnut consumption in a weight reduction intervention: Effects on body weight, biological measures, blood pressure and satiety. Nutr J 16(1): 76.
- Neale EP, Tapsell LC, Martin A, Batterham MJ, Wibisono C (2017) Impact of Providing Walnut Samples in a Lifestyle Intervention for Weight Loss: A Secondary Analysis of the Health Track Trial. Food Nutr Res 61(1): 1344522.
- Chinedu E, Arome D, Ameh FS (2013) A new method for determining acute toxicity in animal models. Toxicol Int 20(3): 224-6.
- (2011) National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals : Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press (US).
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low- density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6): 499-502.
- Farzad N, Mohammadjavad Z, Kamran A, Hossein H Hassan Y (2016) The Effect of Walnut Oil Consumption on Blood Sugar in Patients with Diabetes Mellitus Type 2. Int J Endocrinol Metab. 14(3): e34889.
- Ma Y, Njike VY, Millet J, Dutta S, Doughty K, et al. (2010) Effects of Walnut Consumption on Endothelial Function in Type 2 Diabetic Subjects: A Randomized Controlled Crossover Trial. Diabetes Care 33: 227-232.
- Sánchez González C, Ciudad CJ, Noé V, Izquierdo Pulido M (2017) Health Benefits of Walnut Polyphenols: An Exploration Beyond their Lipid Profile. Crit Rev Food Sci Nutr 57(16): 3373-3383.
- Haddad EH, Gaban Chong N, Oda K, Sabaté J (2014) Effect of a Walnut Meal on Postprandial Oxidative Stress and Antioxidants in Healthy Individuals. Nutr J 13(1): 4.
- Petrović Oggiano G, Debeljak Martačić J, Ranković S, Pokimica B, Mirić A, et al. (2020) The Effect of Walnut Consumption on n-3 Fatty Acid Profile of Healthy People Living in a Non-Mediterranean West Balkan Country, a Small Scale Randomized Study. Nutrients 12(1): 192.
- Camara, CRS and Schlegal (2016) A Review on the Potential Human Health Benefits of the Black Walnut. A Comparison with the English walnut and other Tree Nuts. Molecules 25(19): 4516.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...